Overcome a bottleneck in gene therapy development and manufacturing
While visible and subvisible particle (SVP) analysis is essential for ensuring the quality and safety of all therapeutics, testing adeno-associated virus (AAV) based gene therapies for visible and subvisible particles has been a challenge due to the often limited material available for testing.
The long standing methods for particle analysis—light obscuration and flow imaging—both require large volumes of material for testing, which can be expensive and time-consuming to produce when it comes to AAV.
However, with the Backgrounded Membrane Imaging (BMI) and Fluorescence Membrane Microscopy (FMM) technologies in the Aura GT and Aura+ systems, you can rapidly screen for instability and AAV aggregation in the ultra-low volumes that make sense for gene therapy development.
Why Use Aura Systems for AAV Analytics?
Because you can achieve so much more insight with much less material:
- Detect and characterize particles not measured by dynamic light scattering (DLS) or size exclusion chromatography (SEC)
- Preserve sample, using as little as 5 μL per test
- Quickly screen a wide range of conditions with our 96-well format
- Obtain detailed information on particles that other methods can’t deliver, including size, morphology, count, and size distribution
- Move quickly with an analysis time of about 1 minute per sample
- Evaluate a wide range of particle sizes, measuring 1 μm to 5 mm with high reproducibility
- Achieve high sensitivity because particles are imaged without the interference of buffer or matrix
- See more detail for better particle identification with high-resolution magnification
- Maintain compliance with the option for 21 CFR Part 11 software
Drive Vector Formulation Decisions And Assess Quality
A strong indicator of AAV stability, SVP measurements can deliver data to guide your decision making about viral vector formulation and quality assessment, minimizing risk.
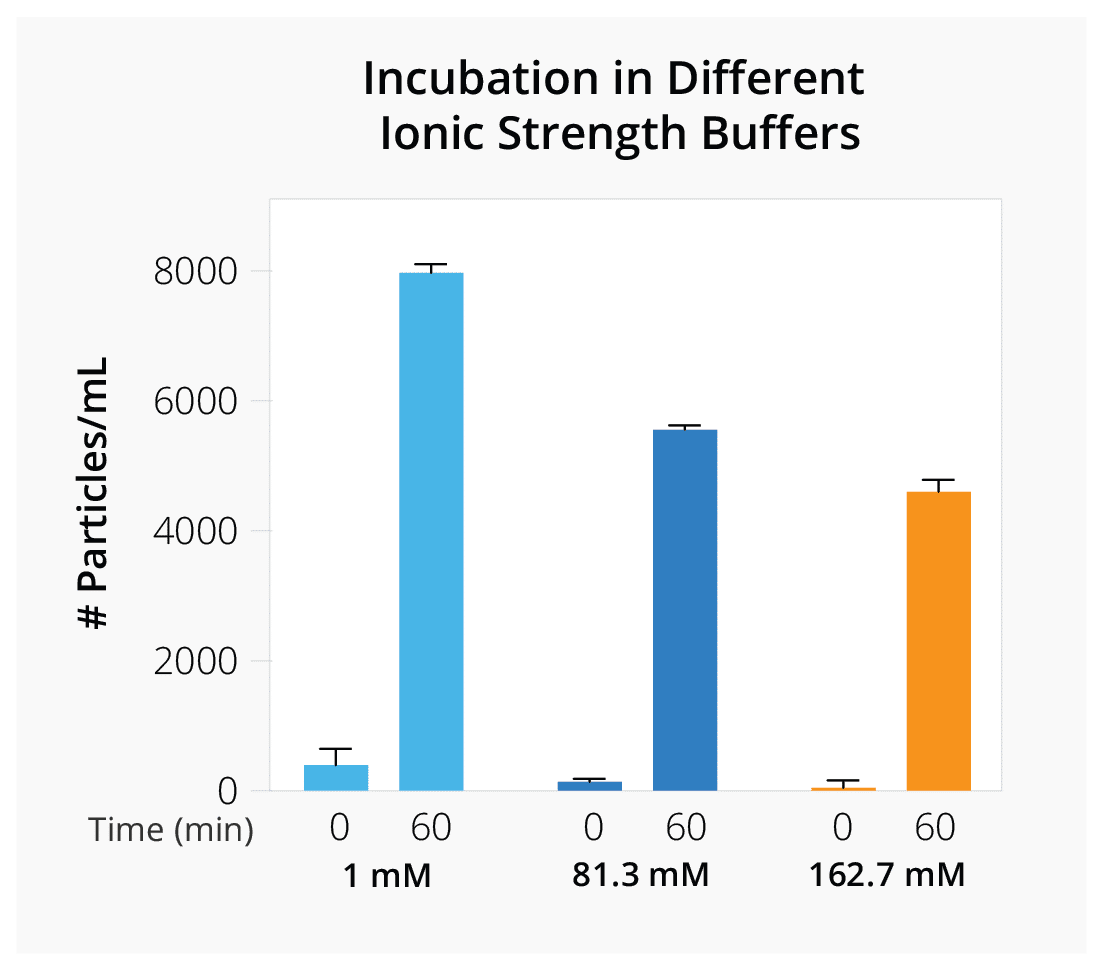
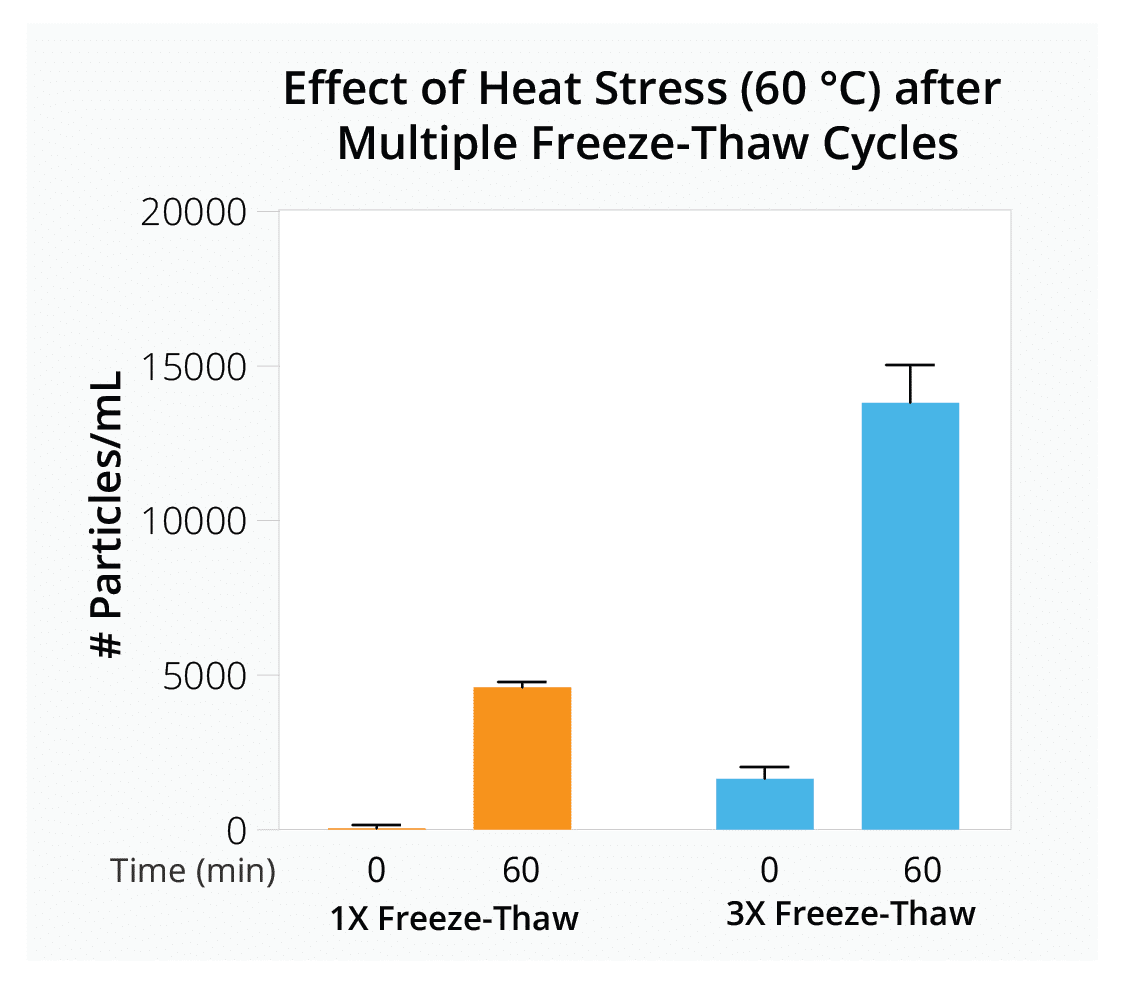
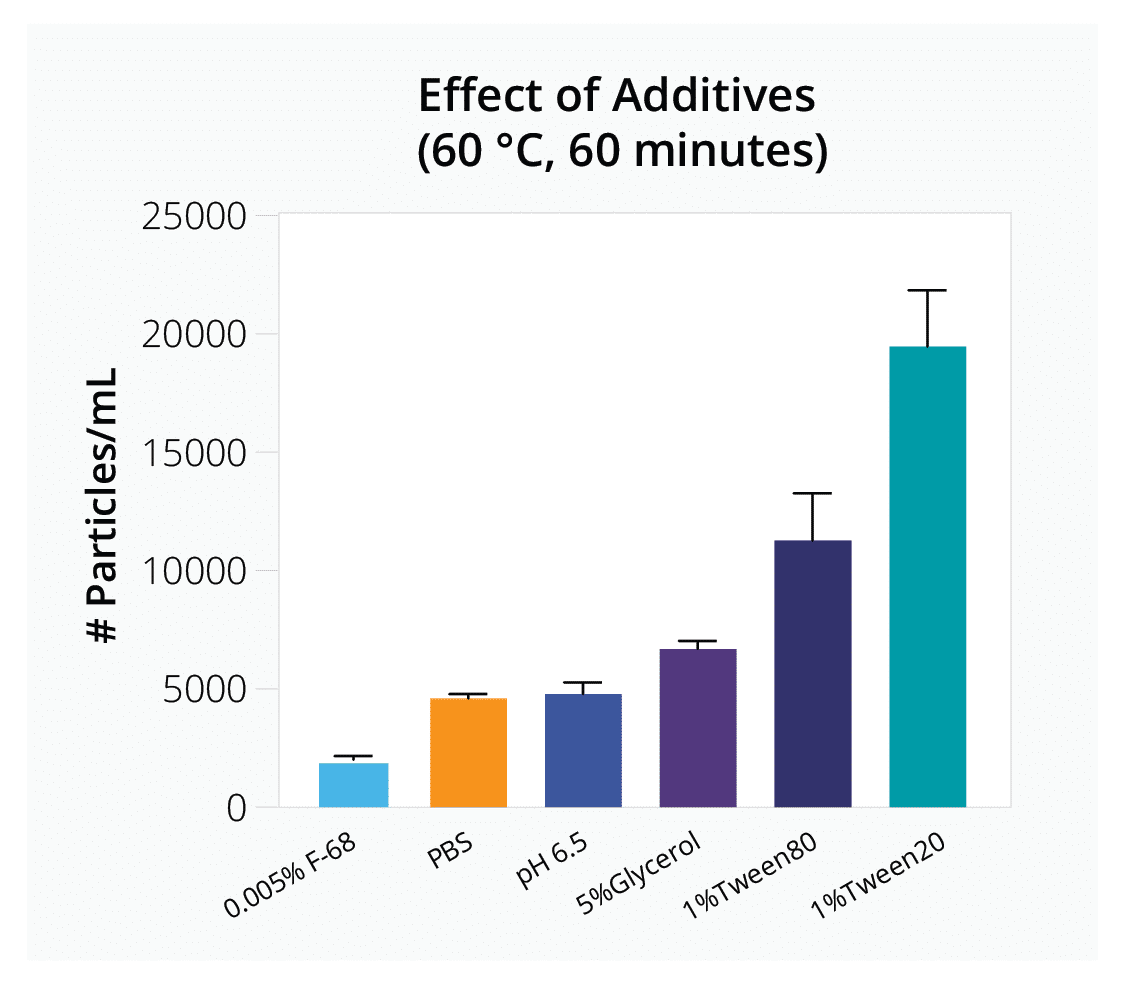
Quickly evaluate how parameters like freeze-thaw cycles, ionic strength, and different additives influence vector stability to determine optimal AAV formulation and storage conditions for your gene therapy product.
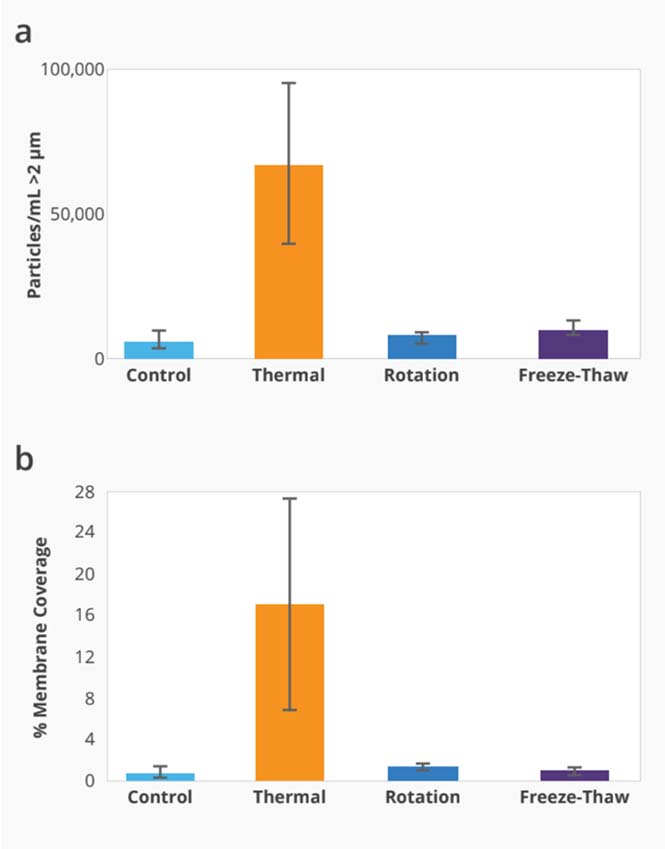
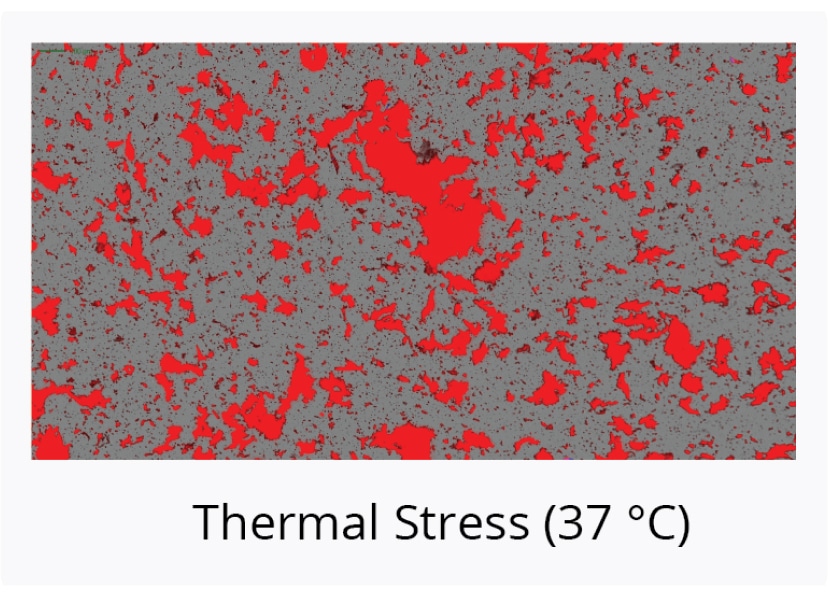
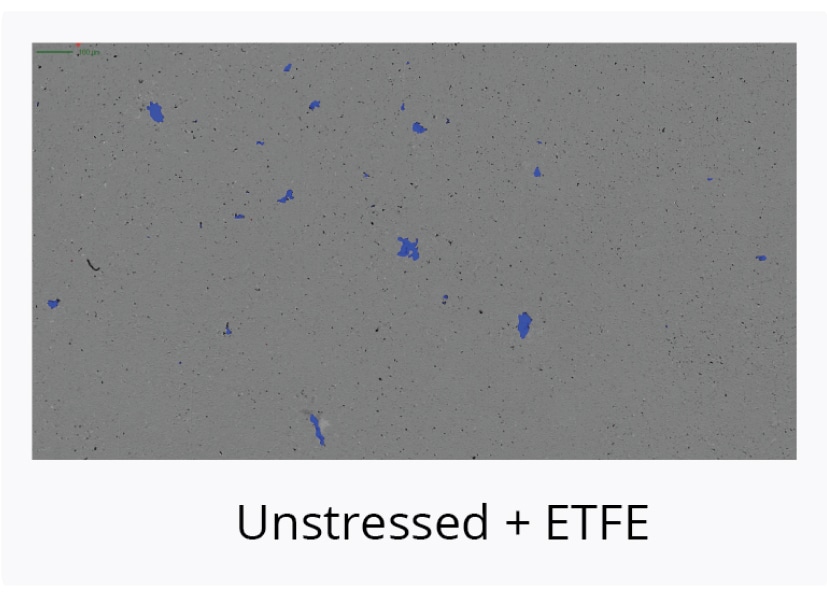
Compare Stability of AAV Serotypes Under Thermal Stress
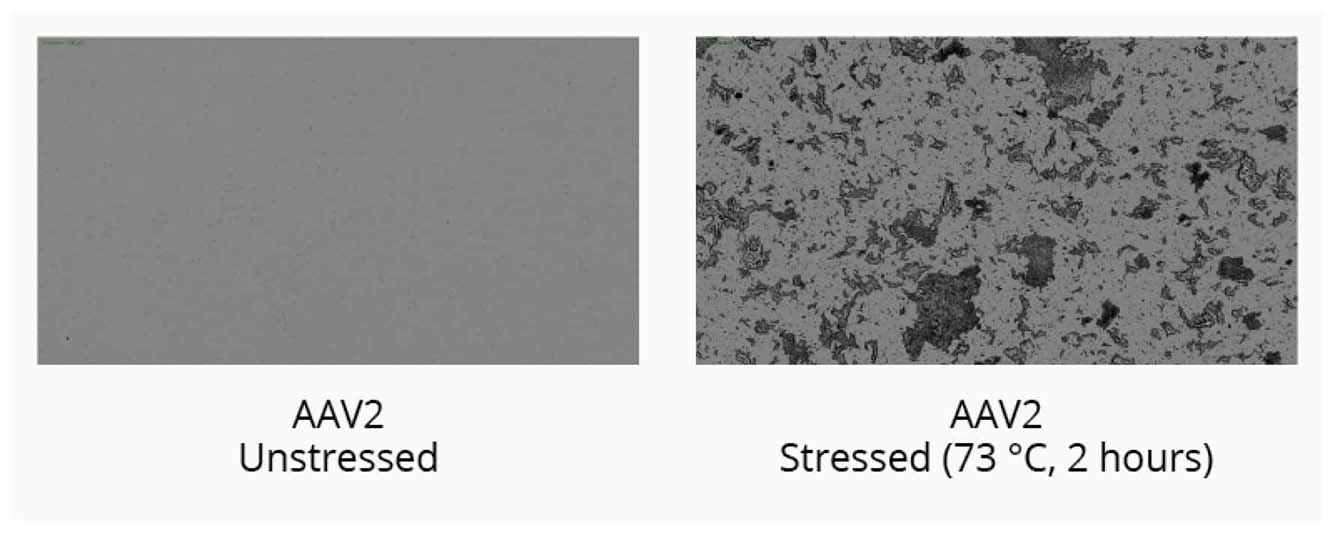
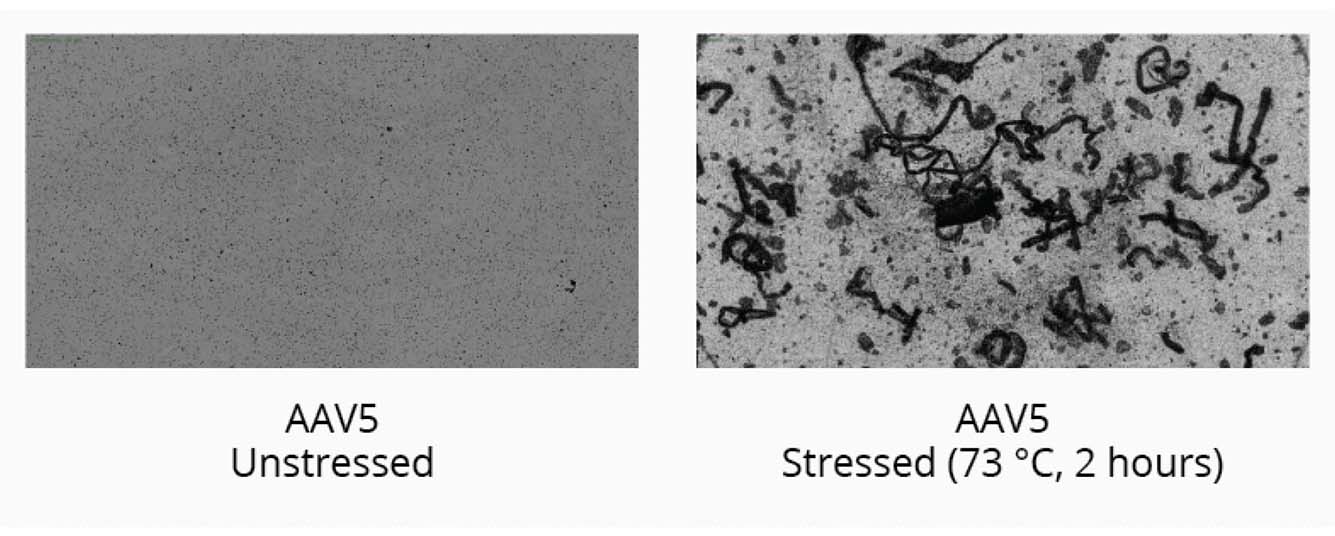
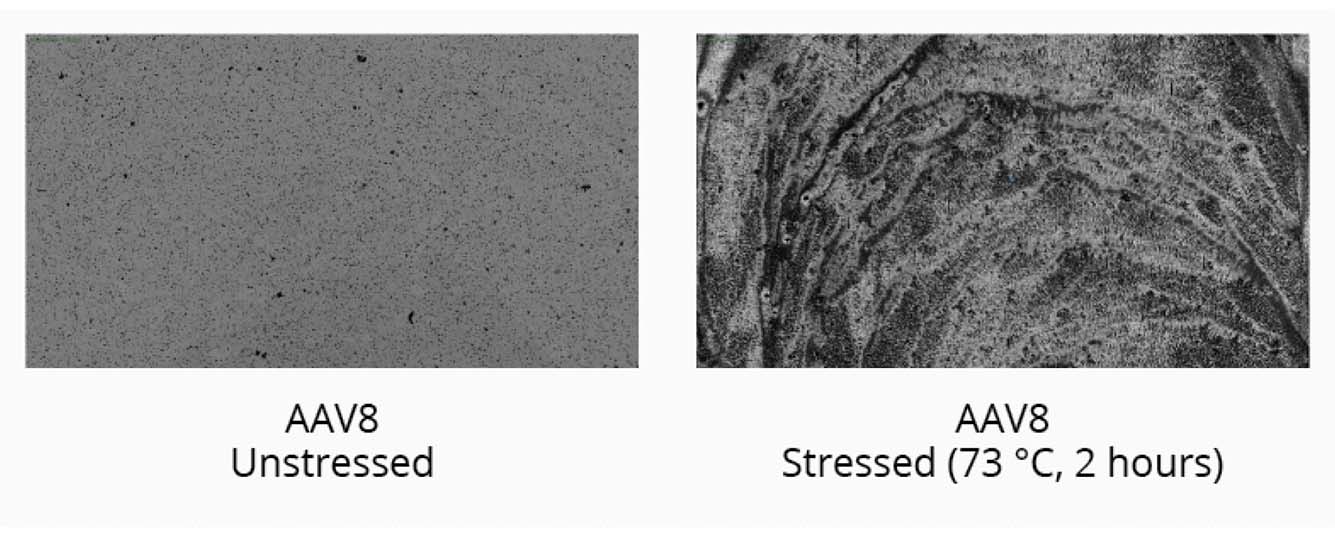
Start your gene therapy development by selecting the best AAV serotype to package your genetic payload. Brightfield imaging using BMI on the Aura GT provides highly resolved images that allow you to compare levels of SVPs in stock samples for different serotypes and categorize the type of aggregate formed for each serotype based on morphology after thermal stress (73°C, 2 hours). The data can then be quantitated using Particle Vue software.
Using the Aura GT system, we observed low levels of SVPs in stock AAV2 samples. Well-defined, small to large granular particles are formed after thermal stress.
We observed increased levels of SVPs in stock AAV5 samples compared to stock AAV2 samples. Large fibrillar structures are formed after thermal stress.
We observed increased levels of SVPs in stock AAV8 samples compared to stock AAV2 and AAV5 samples. Complete sample degradation was observed as the stressed AAV8 sample denatured into a film-like matrix.
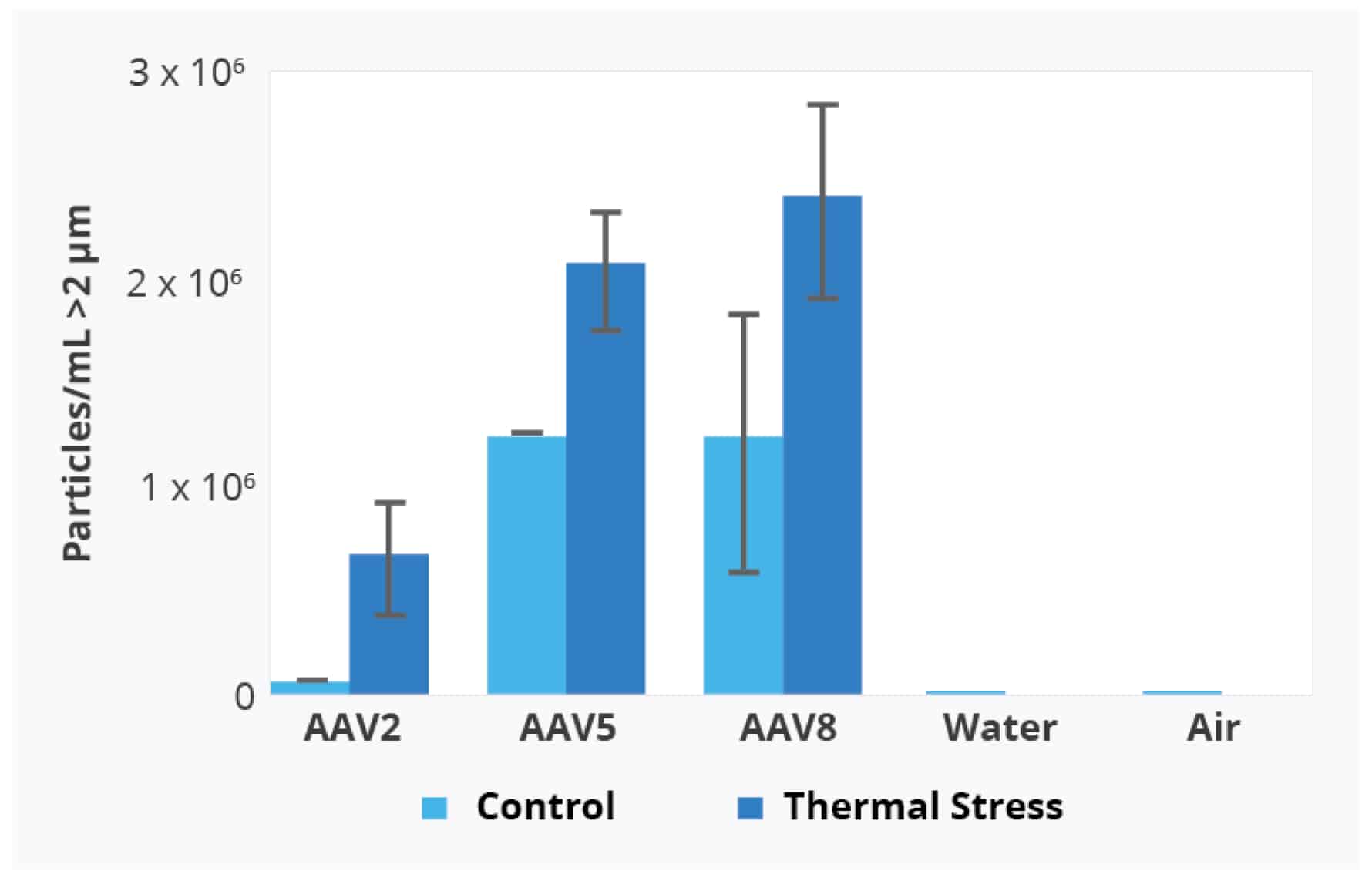
Particle Vue software can provide a quantitative analysis of the different AAV serotypes with and without thermal stress by measuring the concentration of particles > 2 µm.
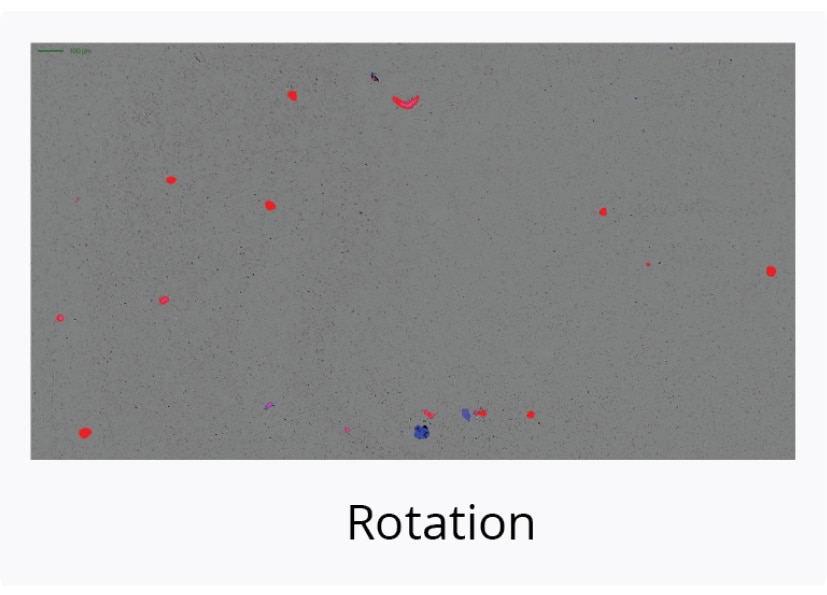
Definitively Confirm Detected SVPs are Aggregated Capsid
Continuing the above study, we selected the AAV2 sample for further analysis because it was the most stable of the three serotypes we investigated.
We subjected the AAV2 sample to additional stressors and then used the FMM capabilities of the Aura GT system to identify the particles.
We stained the AAV2 sample with thioflavin T (ThT), a fluorescent dye that specifically binds to aggregated protein but not non-protein particles that can be derived from sources like plastic, rubber, or degraded additives, and determined that thermal stress resulted in a high degree of capsid aggregation.
Featured Products
Frequently Asked Questions
There are numerous AAV (Adeno-associated virus) serotypes that have been identified and characterized to date, with new serotypes continuously being discovered. As of the latest research, over 100 different AAV serotypes and variants have been identified, each exhibiting unique properties such as tissue tropism, transduction efficiency, and immunogenicity. These serotypes have diverse applications in gene therapy and research, offering potential vectors for delivering therapeutic genes to target tissues and organs.
AAVs (Adeno-associated viruses) are typically manufactured using recombinant DNA technology and cell culture techniques. The manufacturing process involves several key steps:
- Vector Design: AAV vectors are engineered to contain the therapeutic gene of interest within the viral genome.
- Cell Culture: Host cells, such as HEK293 cells or insect cells, are grown and maintained in bioreactors under controlled conditions.
- Transfection: Plasmids containing the AAV vector genome and helper virus genes required for AAV replication are inserted into host cells.
- AAV Production: The transfected cells create AAV particles, which are harvested from the cell culture supernatant.
- Purification: AAV particles are separated from cellular debris and contaminants using chromatography or other purification techniques.
- Formulation: The purified AAV particles are made into the final vector product for research or therapeutic use.
The manufacturing process is highly regulated and requires stringent quality control measures to ensure the safety, purity, and efficacy of the AAV vectors.