Utilizing Surface Tension to Predict Surfactant Formulation Requirements for End-to-End Stability
July 11, 2024
Biological drug products are a complex mix of elements, each playing a key role in maintaining the product’s stability throughout its shelf life. One such crucial element is surfactants. These substances, primarily polysorbates, are added to drug formulations to stabilize air-water interfaces—which is known to induce protein aggregation.
However, the journey of these drug products extends beyond the shelf. It continues into their clinical use—from dosing to administration—and it’s here that the challenge of maintaining stability becomes even more significant. This is particularly true for large-molecule drug products that are typically administered via IV infusion.
The Need for Stability Analysis in Early Formulation Development
Early formulation development predominantly emphasizes preserving protein conformation and colloidal stability over the drug product’s shelf life. However, this approach often overlooks stability during dose preparation and administration.
For instance, preparing an IV bag exposes the therapeutic protein to a different solution environment. This process dilutes the stabilizing excipients originally added to the drug product formulation. Furthermore, the dynamic changes mixing in IV bags can induce in the air-water interfacial area may cause protein aggregation if not adequately protected.
Therefore, understanding surfactant requirements for end-to-end stability in early formulation development is critical. It provides invaluable insights for drug product formulation and ensures robust clinical preparation.
Exploring the Impact of Polysorbates on Protein Aggregation
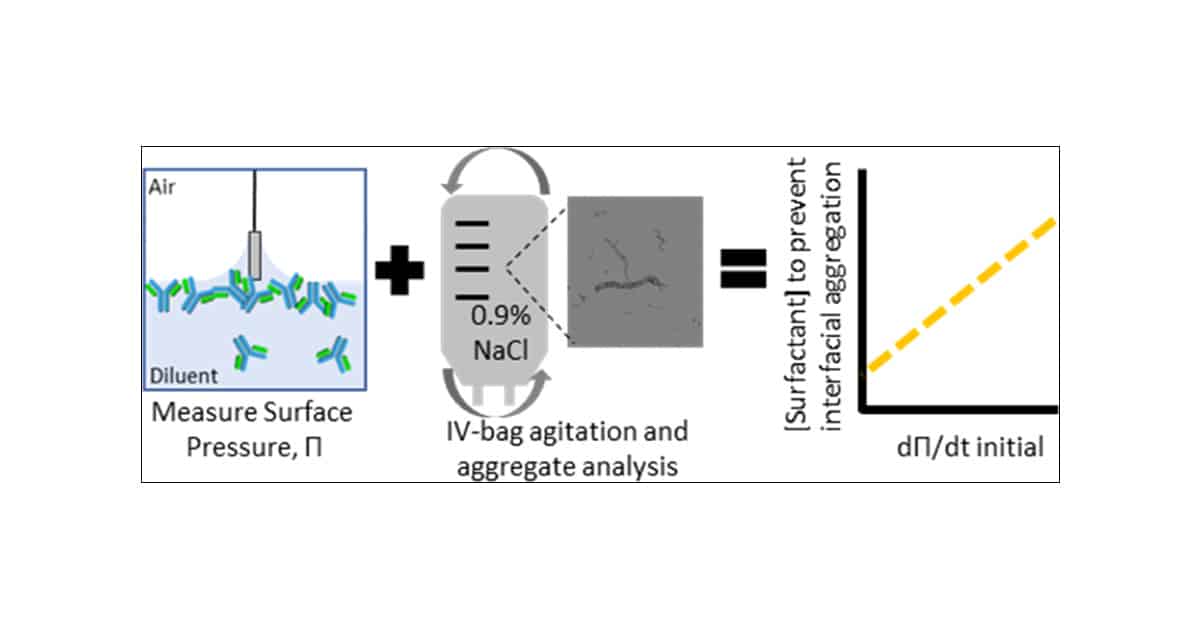
A recent study examined the impact of polysorbates on protein aggregation during IV-bag dilution. The research aimed to determine if changes in surface tension could predict surfactant formulation requirements for end-to-end stability.
In the study, the interfacial properties of five proteins in 0.9% saline and dextrose were measured. Shaking studies were conducted to identify the minimum surfactant concentration required to prevent the formation of subvisible and visible particles in each diluent.
Researchers employed a range of techniques, including Halo’s backgrounded membrane imaging (BMI), microflow digital imaging, and SE-HPLC, to thoroughly characterize aggregation. They measured the aggregate distribution for the most surface-active protein, demonstrating that it was a function of the surfactant concentration.
Interestingly, the study found that bulk protein properties provided limited insight as a predictive method for interfacial stability. However, the team found a correlation to predict the surfactant concentration required for IV-bag stability in each diluent based on the interfacial properties of the protein. They formulated a mathematical model to determine how much surfactant is needed for different solutions to stop proteins from clumping, based on how the protein behaves at the surface.
Utilizing BMI to Link Protein Behavior to Shaking
Among the techniques used, the study employed Halo Labs’ backgrounded membrane imaging (BMI) to quantify subvisible particle size and distribution.
BMI is a technique that stands out for its precision and sensitivity, matching or surpassing current flow imaging techniques. BMI has an advantage over other methods for quantifying subvisible particles due to its high-throughput nature, simplicity, speed, and low sample volume requirement. Employing BMI during the initial stages of development can result in significant time and cost savings.
Conclusion
This study has opened up new horizons thanks to the development of a tool that can predict how drugs will react to shaking during preparation and storage.
The team studied how proteins behave at the surface of liquids, as shaking can cause proteins to clump together. Using advanced imaging techniques, such as BMI, they were able to link protein behavior to shaking results and create a model to predict how much stabilizer (surfactant) is needed to prevent clumping. This model’s versatility allows it to be applied to various solutions used in drug preparation, taking into account shaking intensity and container shape.
As we gain a deeper understanding of protein behavior, we move closer to designing drugs that maintain their safety and effectiveness throughout their lifecycle.
Related
- See how BMI works
- Differentiate between total protein aggregation vs. specific protein aggregation using the Aura immunoassay
- Surprised IV bags can induce aggregation? Filtering your product can too
Reference
1. Vargo, K.B., Stahl, P., Hwang, B., Hwang, E., Giordano, D. et al. Surfactant Impact on Interfacial Protein Aggregation and Utilization of Surface Tension to Predict Surfactant Requirements for Biological Formulations. Molecular Pharmaceutics 2021 18 (1), 148-157. DOI: 10.1021/acs.molpharmaceut.0c00743
DISCOVER THE AURA FAMILY
Read More ON this topic