96-well plate format
5µL sample volume
1 minute assay
Subvisible Particles Are Important to Small Molecule Drugs
Solubility of an active ingredient for small molecule development is just as important as subvisible particles (SVPs). The particle size can affect the solubility of the drug itself.
Aura+ with Backgrounded Membrane Imaging (BMI) enables high throughput screening to characterize and understand how the distribution of all particles and its respective sizes impact the solubility of the drug. Small molecule drug developers can now quickly identify the best conditions and concentrations required to maintain compound solubility without sacrificing precious sample or time.
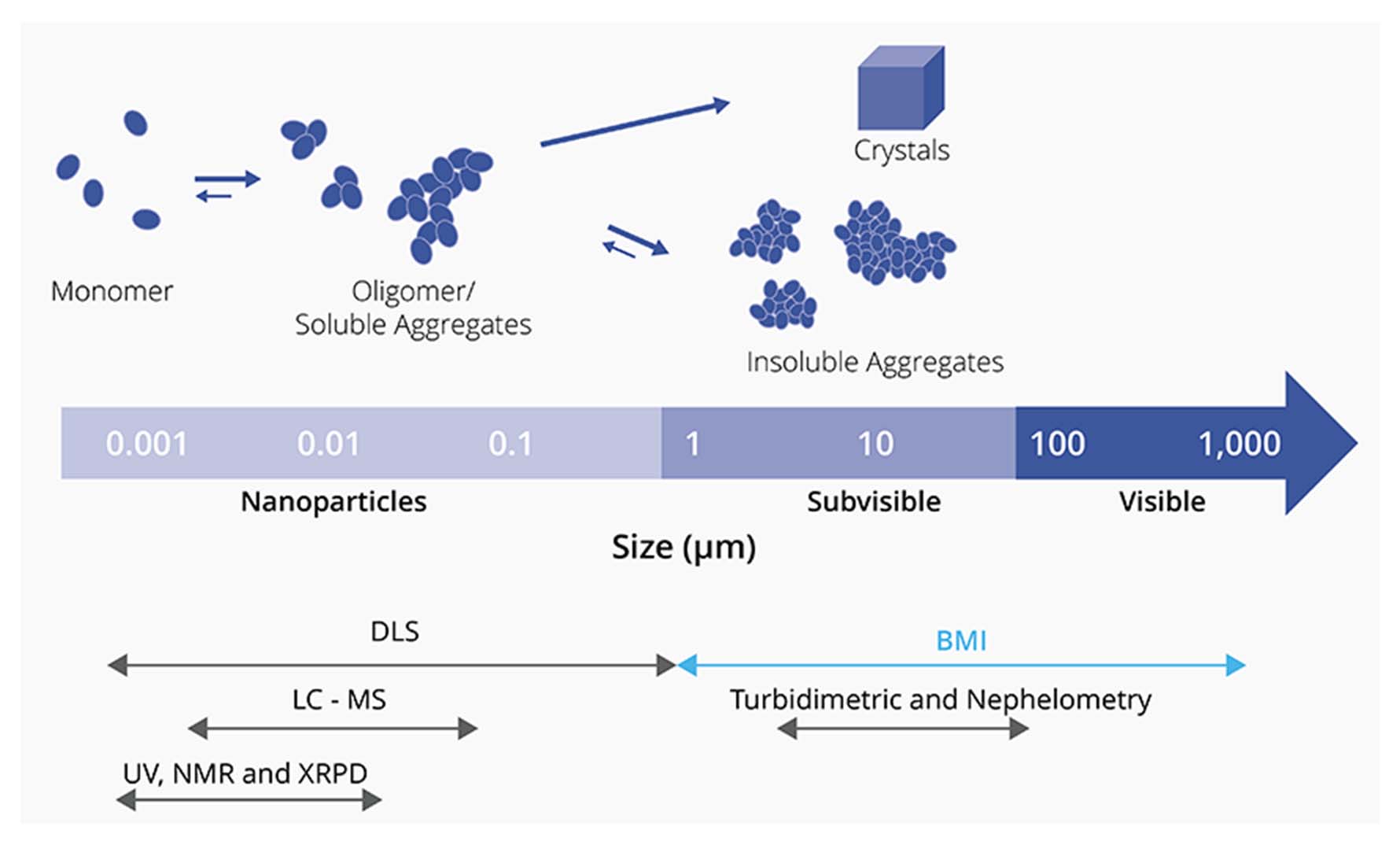
Analysis for wide range of particle sizes with BMI.
More Sensitive Kinetic Solubility Measurement
To demonstrate correlation of subvisible particle counts measured on Aura systems to solubility changes, four control compounds with different aqueous solubilities were selected for analysis with BMI. A concentration series for each compound was prepared directly from DMSO stocks, then diluted into PBS, pH 7.4 to 1% DMSO final concentration. Pharmaceutical companies are increasingly utilizing these methods to enhance their understanding of compound behavior in solution.
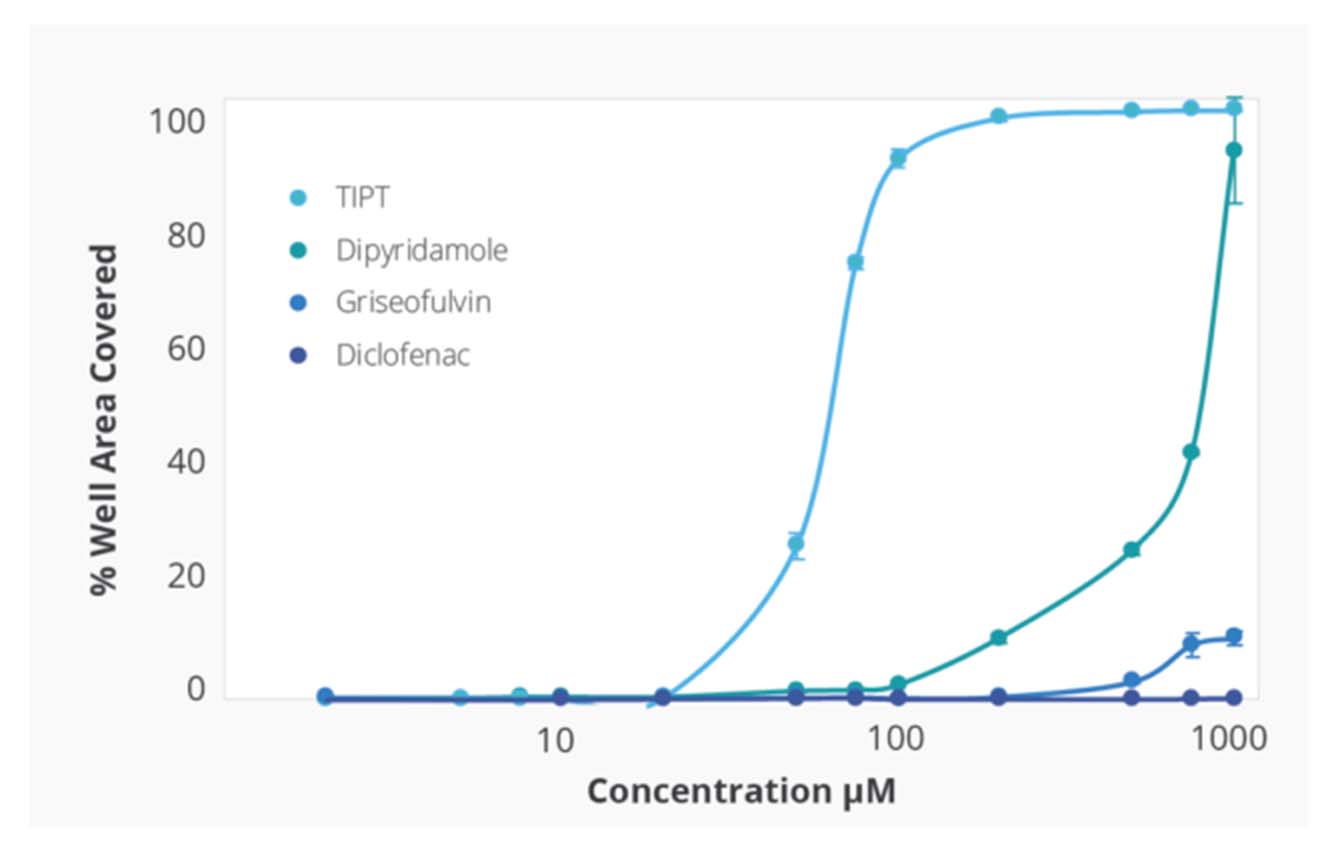
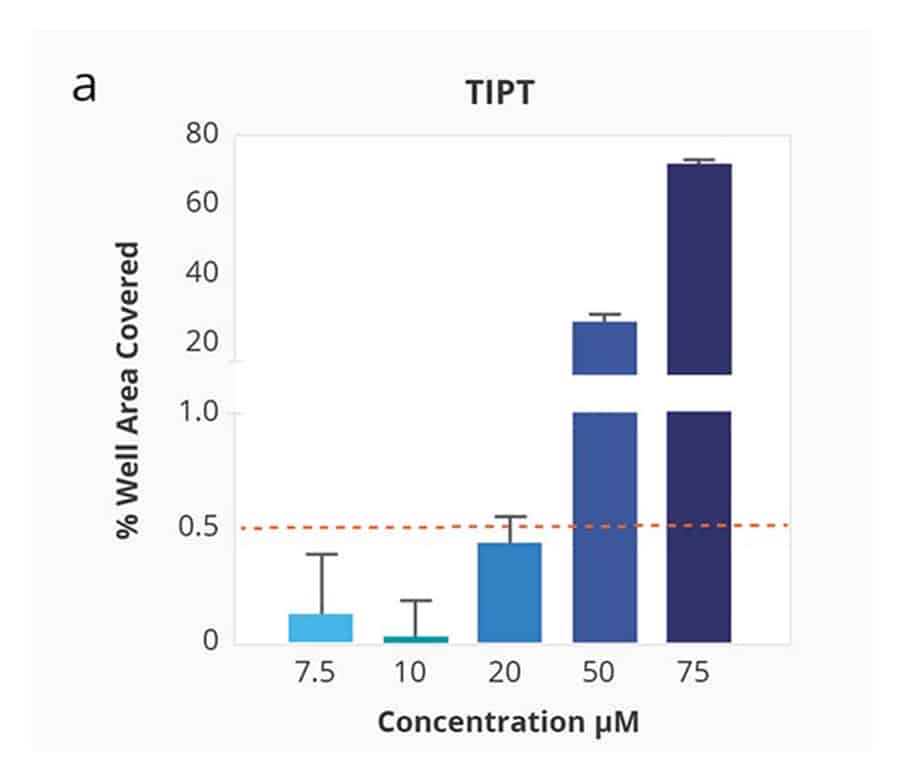
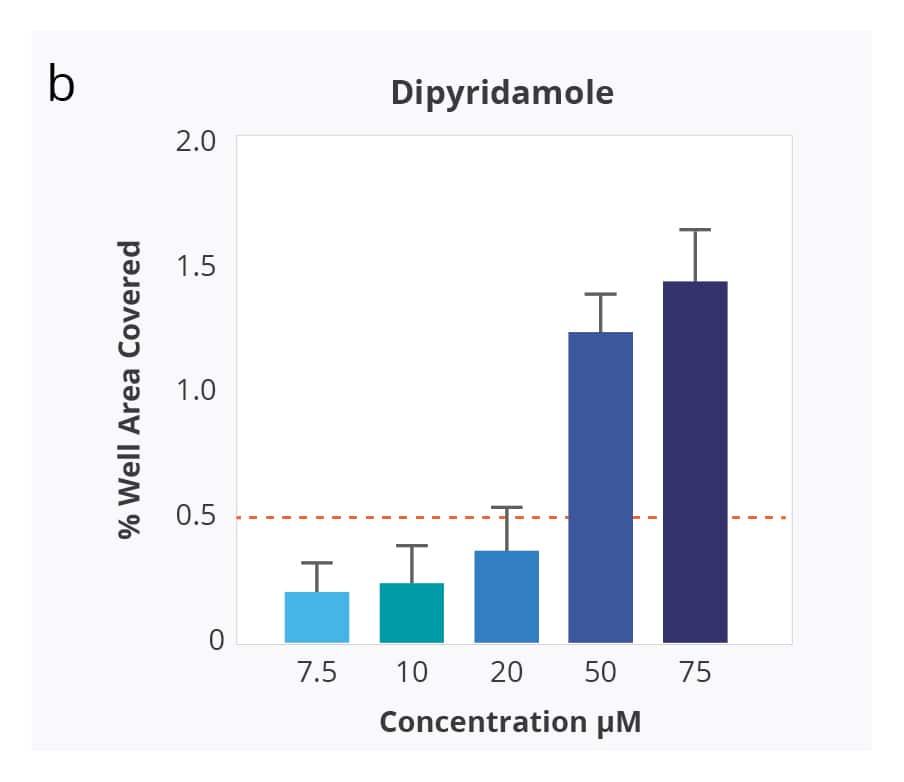
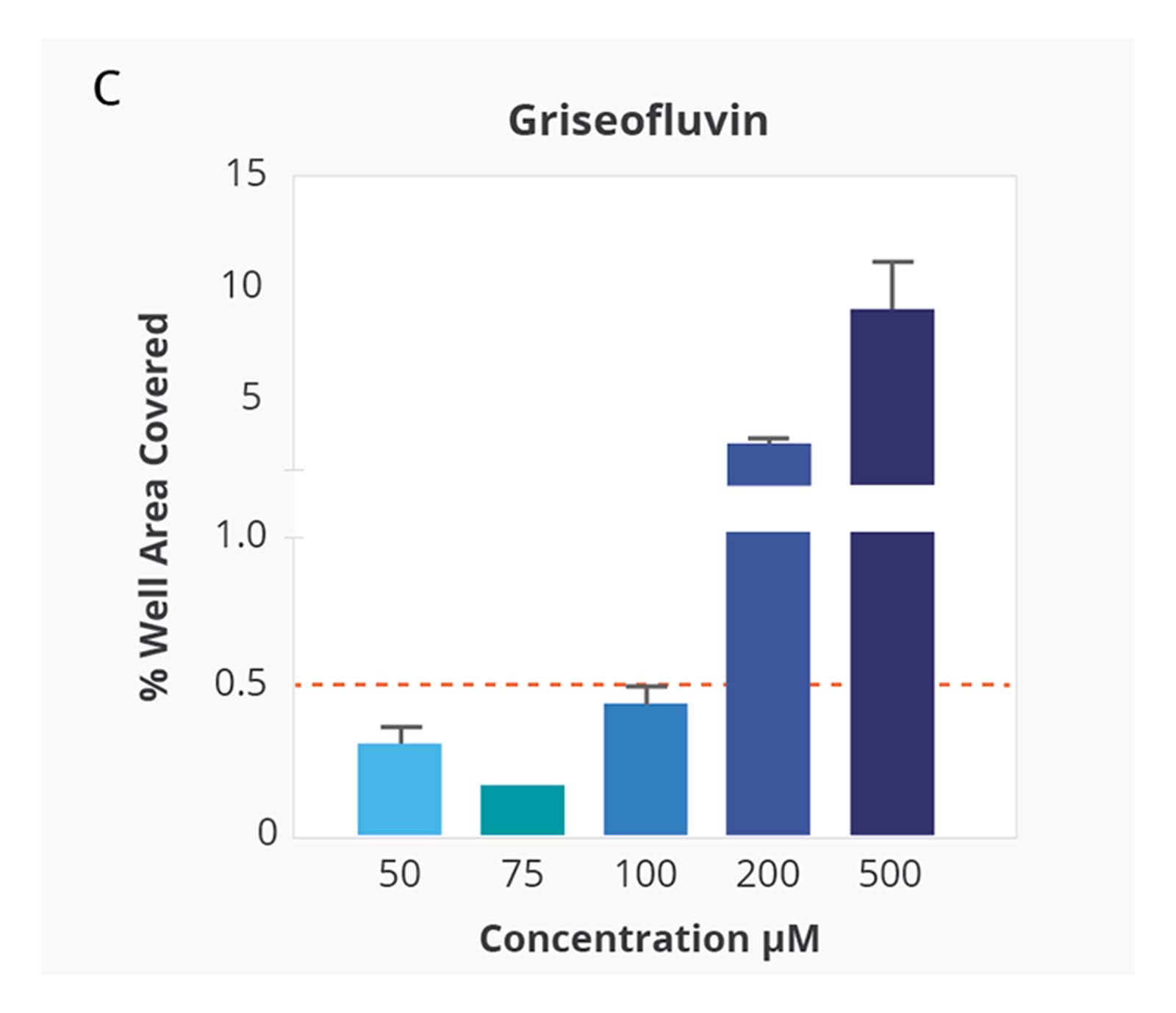
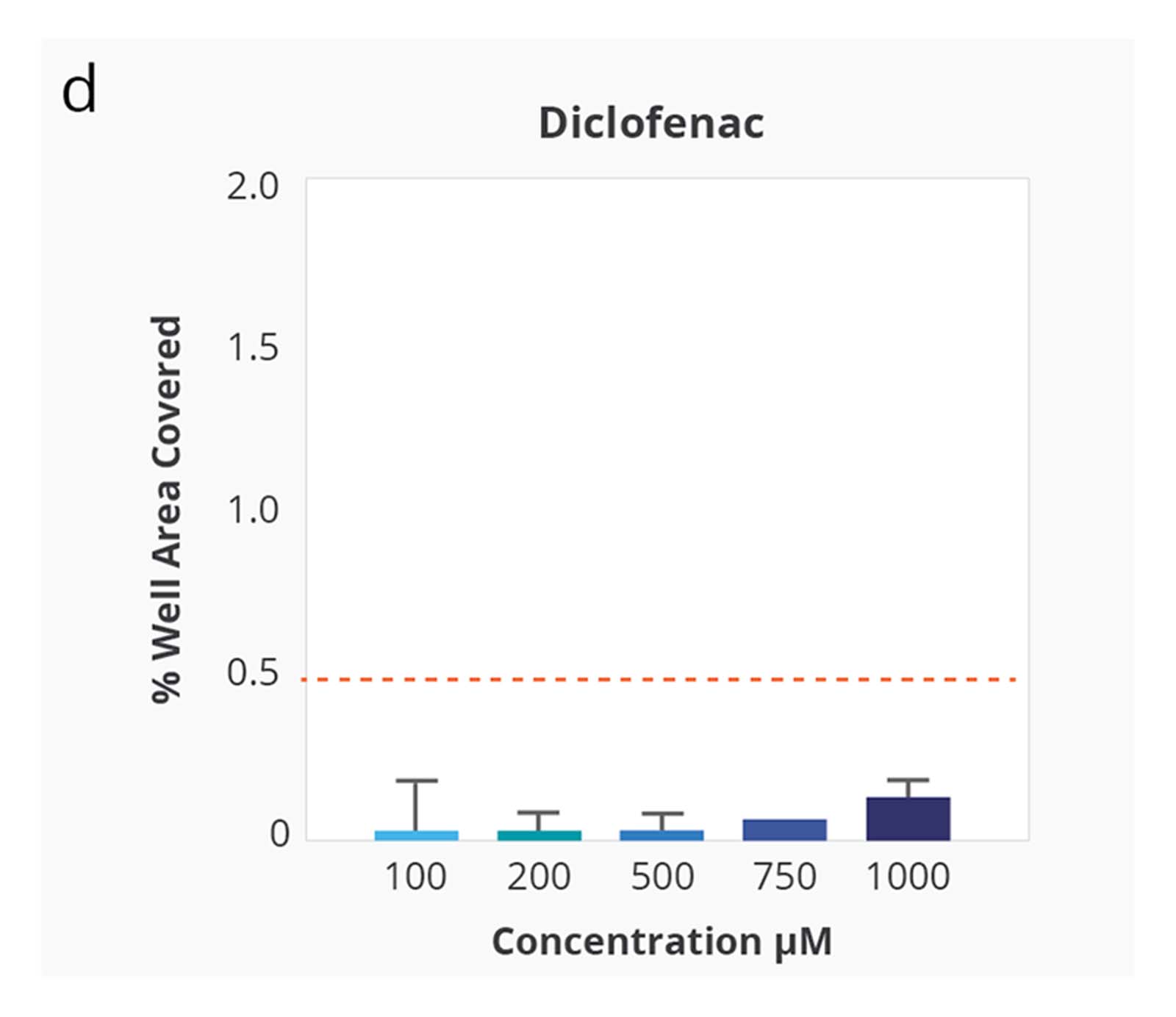
Compound Solubility Profiles on Aura. Relationship between increasing concentration in PBS 7.4 and surface coverage of the membrane well with insoluble particles. Differing compound profiles support emergence of particle aggregates as an indicator of solubility of the different compounds. Of note is that the inflection of the particle coverage curves and rate of increased precipitate formation vary for each compound.
Kinetic solubility range for each compound was reported as the concentration above and below where membrane area coverage by particles exceeded a baseline threshold. The midpoint of this range was assigned as the compound’s estimated solubility.
Ranking of the four test compounds for solubility with the Aura system was identical to ranking by turbidimetry. However, with Aura particle aggregates were detected at 5–10 times lower compound concentration.
The Value of the Unseen
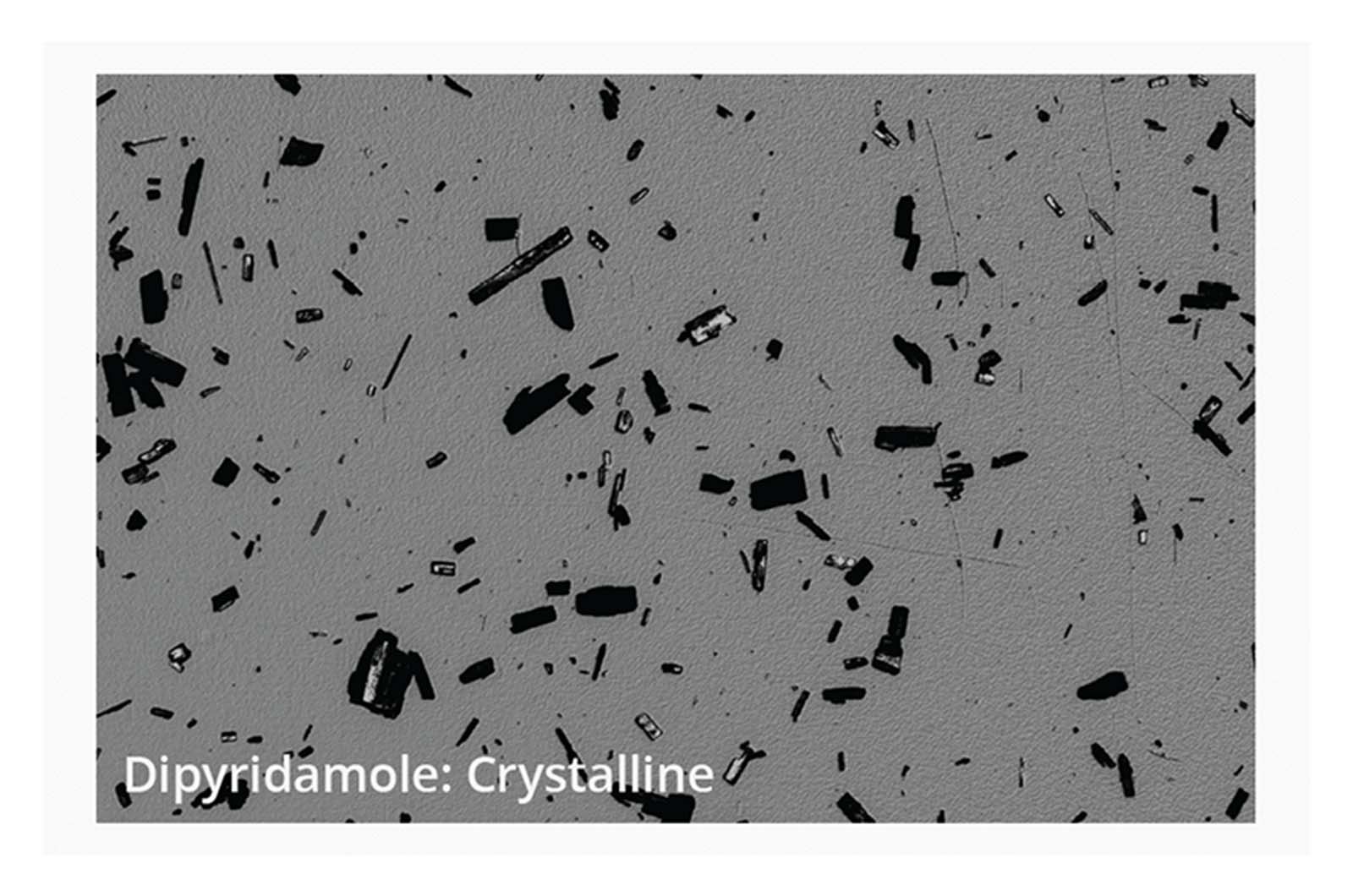
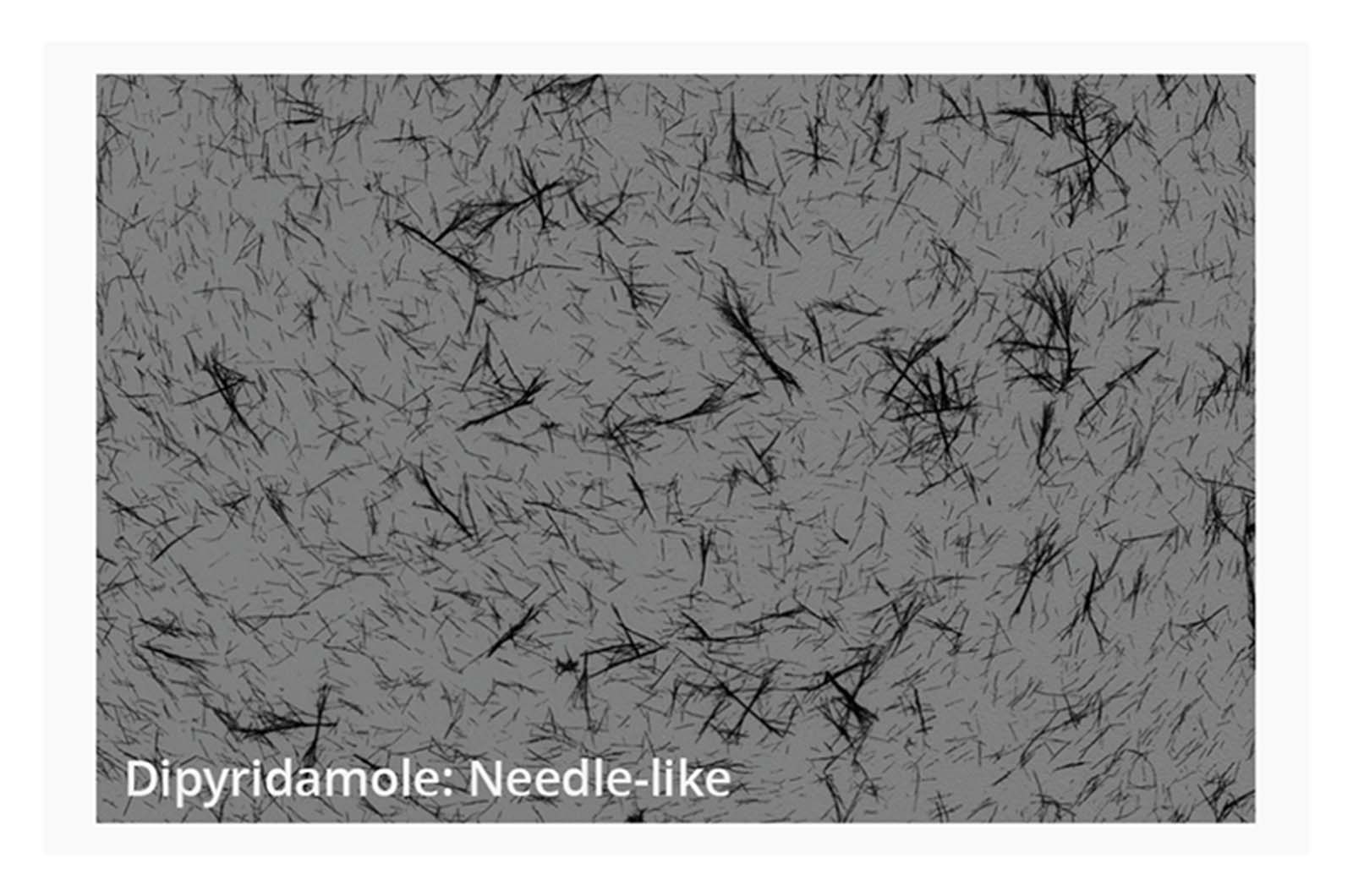
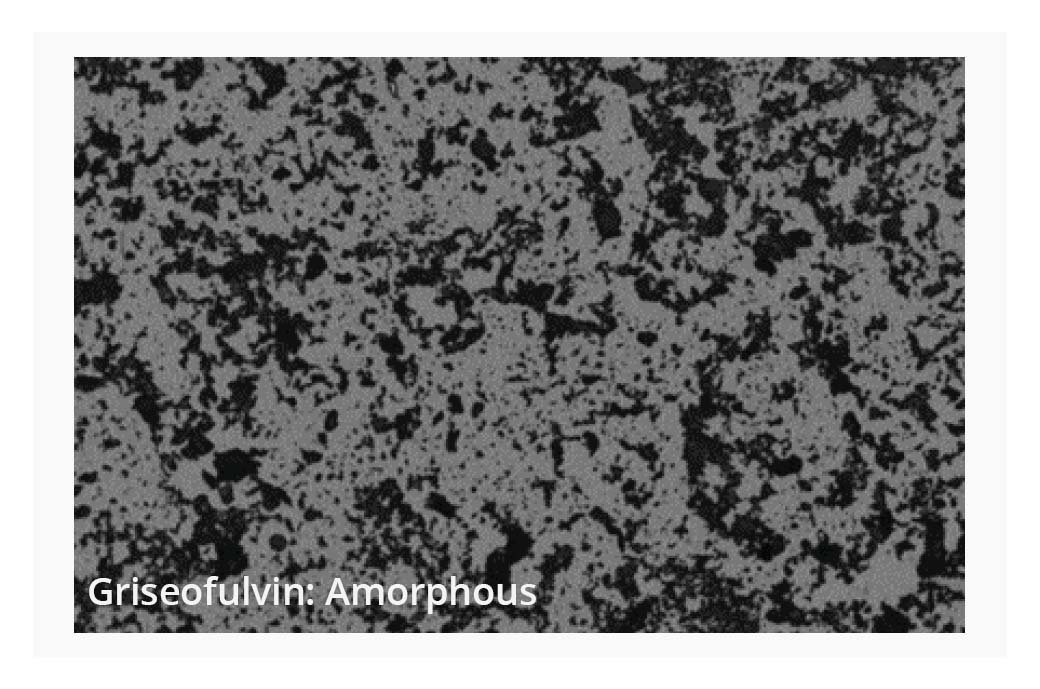
Small scale synthesis of compounds during the selection stage can lead to variation of physical form, which can have a dramatic effect on the measured aqueous solubility of compounds. BMI provides high-resolution images and digital image analysis of aggregate particles to aid in the evaluation of solid-state form. Understanding these variations is crucial, as they influence the building blocks needed for successful compound development and optimization.
Featured product
Frequently Asked Questions
Small molecule screening, also known as high-throughput screening (HTS), is a drug discovery approach that involves testing large compound libraries to identify potential lead compounds with therapeutic activity against specific molecular targets or diseases. Small molecule screening assays typically use automated robotic systems to rapidly screen thousands to millions of compounds against biological targets or cellular models in a high-throughput manner. Screening assays may involve biochemical assays, cell-based assays, or phenotypic assays tailored to the target or disease of interest. Small molecule screening enables the identification of hit compounds that modulate target activity or exhibit desired biological effects, providing starting points for lead optimization and drug development. This approach accelerates the drug discovery process, facilitates the identification of novel drug candidates, and expands the chemical space for therapeutic intervention in various diseases. Incorporating DNA encoded libraries into the screening process can further enhance the efficiency and diversity of compound identification.
Small molecule and biologics development represent two distinct approaches to drug discovery and development based on different types of compounds and manufacturing processes. Small molecule drugs are chemically synthesized organic compounds with low molecular weight (<900 Daltons), whereas biologics are large, complex molecules derived from living organisms, such as proteins, peptides, antibodies, or nucleic acids. Amino acids often serve as the building blocks for biologics, contributing to their complex structures and functions. Small molecule drugs typically target intracellular enzymes, receptors, or ion channels, whereas biologics target extracellular or cell surface proteins, typically via binding interactions. Small molecule drugs are orally bioavailable and can be synthesized using chemical methods, while biologics are often administered via injection and produced using recombinant DNA technology in living cells. Small molecule drugs have simpler chemical structures, lower production costs, and shorter development timelines compared to biologics, which require specialized manufacturing processes, regulatory considerations, and quality control measures. Despite these differences, both small molecule and biologics development play critical roles in drug discovery and offer diverse therapeutic options for various diseases.
Small molecules in drug discovery and development are characterized by several criteria that distinguish them from other types of compounds and pharmaceutical agents. Key criteria for small molecules include:
- Low molecular weight: A molecular weight below 900 daltons, allowing them to penetrate cell membranes, exhibit favorable pharmacokinetic properties, and be orally bioavailable.
- Simple chemical structure: Relatively simple chemical structures composed of carbon, hydrogen, oxygen, nitrogen, and occasionally other elements, facilitating chemical synthesis, modification, and optimization.
- Drug-like properties: Properties such as solubility, stability, permeability, and selectivity, enabling them to interact with specific biological targets and exert pharmacological effects.
- Synthetic accessibility: Can be synthesized using chemical methods from commercially available starting materials or intermediates, allowing for efficient production, scalability, and chemical diversity.
- Oral bioavailability: Often administered orally and can be absorbed through the gastrointestinal tract, reaching systemic circulation and target tissues to exert therapeutic effects.
- Target specificity: Bind to specific molecular targets, such as enzymes, receptors, or ion channels, modulating their activity or function to achieve therapeutic outcomes.
- Pharmaceutical development: Rigorous pharmaceutical development processes, including lead identification, optimization, preclinical studies, and clinical trials, to demonstrate safety, efficacy, and regulatory compliance for market approval.
Overall, these criteria define small molecules as versatile and widely used compounds in drug discovery and development, offering numerous advantages for therapeutic intervention in various diseases.