Don't Settle for Flow Imaging
Protein stability and preventing aggregation are crucial for protein formulation development, but current subvisible analysis techniques may only be giving you part of the story.
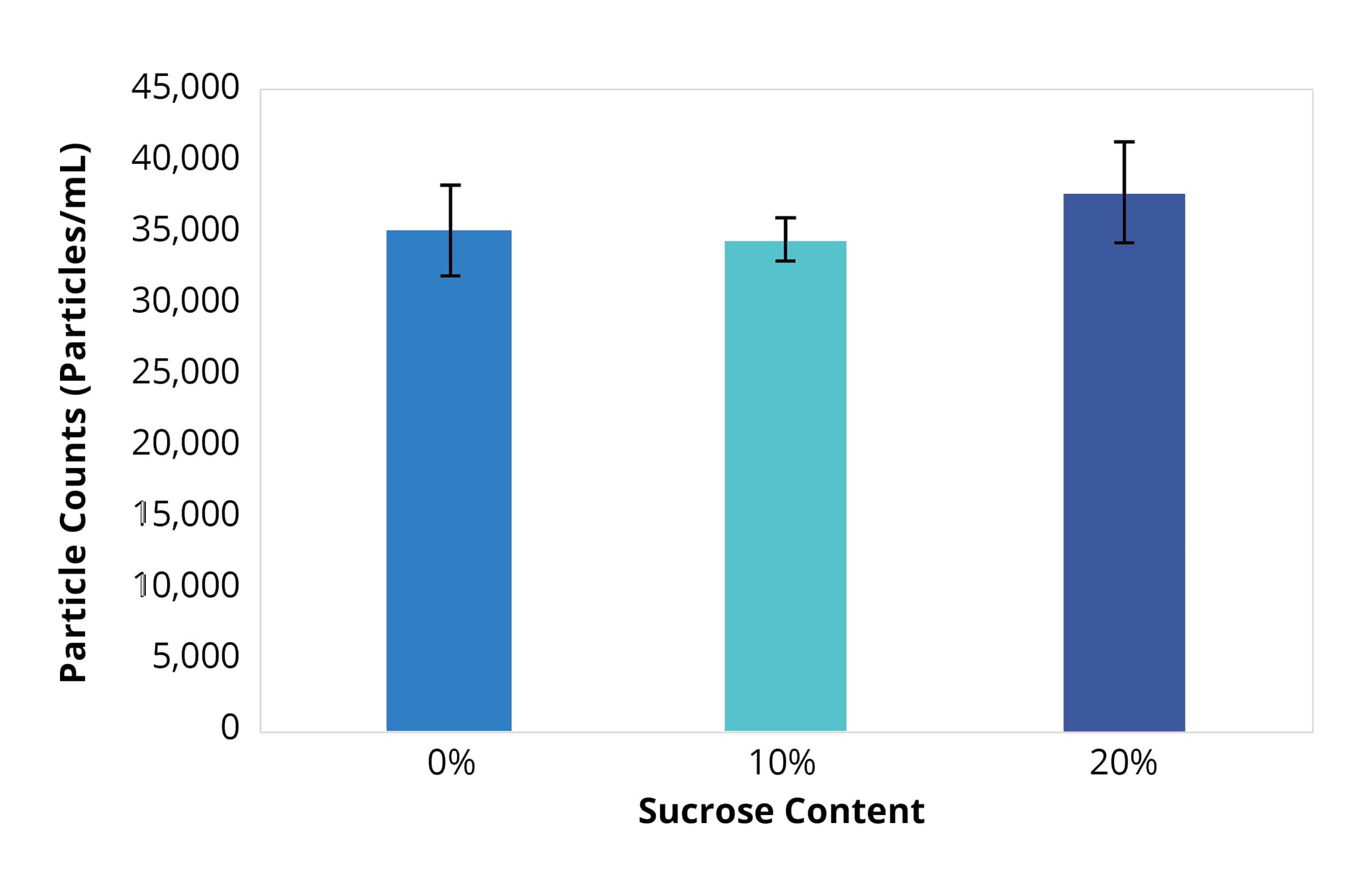
As biologic formulations reach higher levels of concentration, traditional particle analysis methods that require larger sample sizes may become unreliable when assessing subvisible aggregates due to the limited availability of material.
That's why it's time to leave flow cell imaging behind.
Why Use Aura for Protein Formulation Development?
Because you can achieve so much more insight with much less material:
- Detect and characterize particles not measured by dynamic light scattering (DLS) or size exclusion chromatography (SEC)
- Preserve sample, using as little as 5 μL per test
- Quickly screen a wide range of conditions with our 96-well format
- Obtain detailed information on particles that other methods can’t deliver, including size, morphology, count, and distribution
- Move quickly with an analysis time of about 1 minute per sample
- Evaluate a wide range of particle sizes, measuring 1 μm to 5 mm with high reproducibility
- Achieve high sensitivity because particles are imaged without the interference of buffer or matrix
- See more detail for better particle identification with high-resolution magnification
- Meets USP 787 recommendations
- Maintain compliance with the option for 21 CFR Part 11 software
Distinguish Proteins and Non-Proteins With Innovative Technology
Fluorescence Membrane Microscopy (FMM), exclusively on Aura, builds on Backgrounded Membrane Imaging (BMI) to identify, categorize, and further scrutinize particles in your sample by using fluorescent labels. This means you can obtain protein/non-protein ID for a whole 96-well plate assay down to a single individual particle in less than 90 minutes.
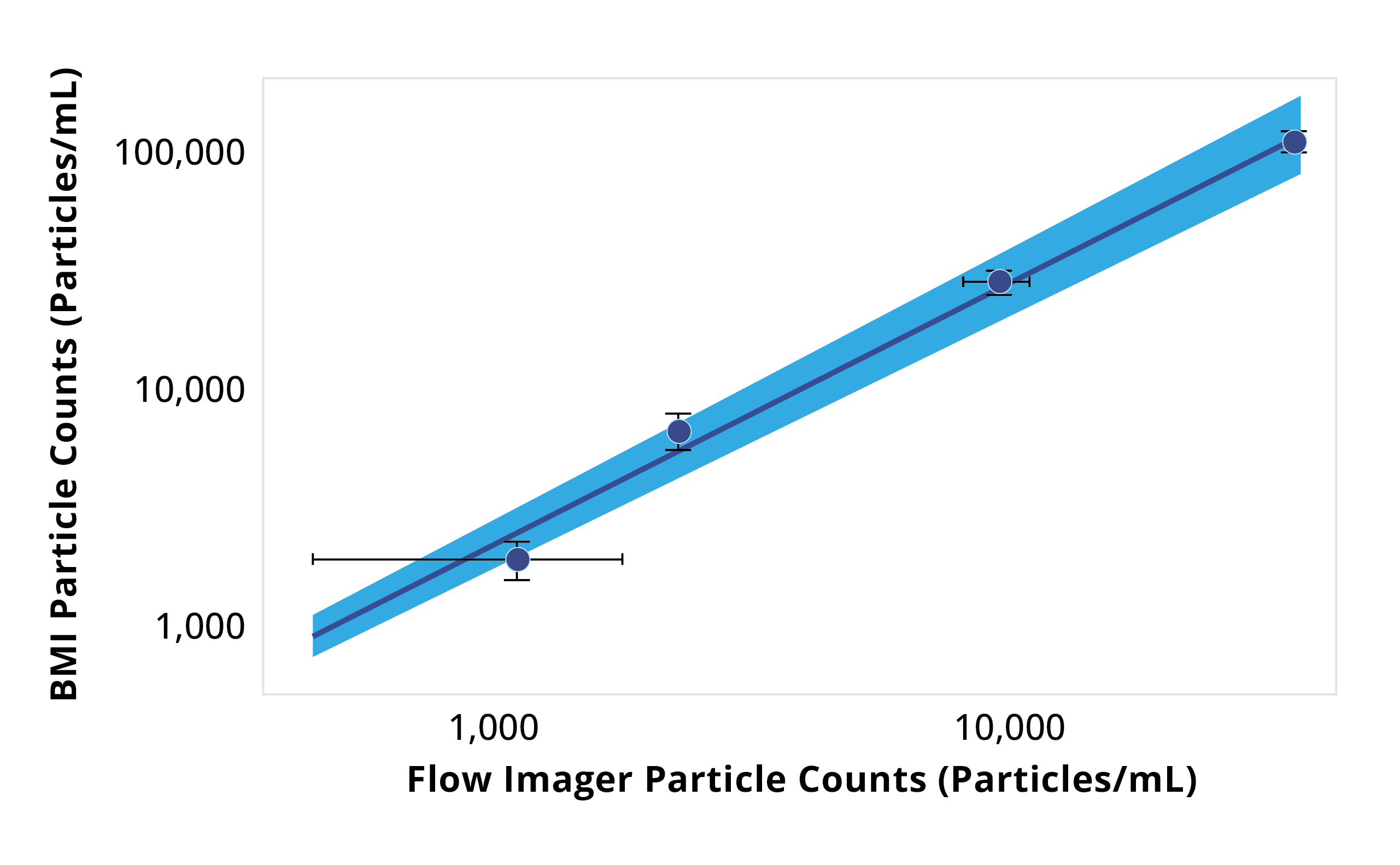
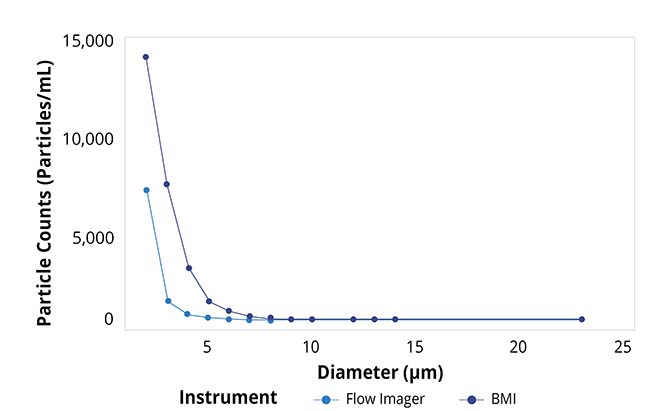
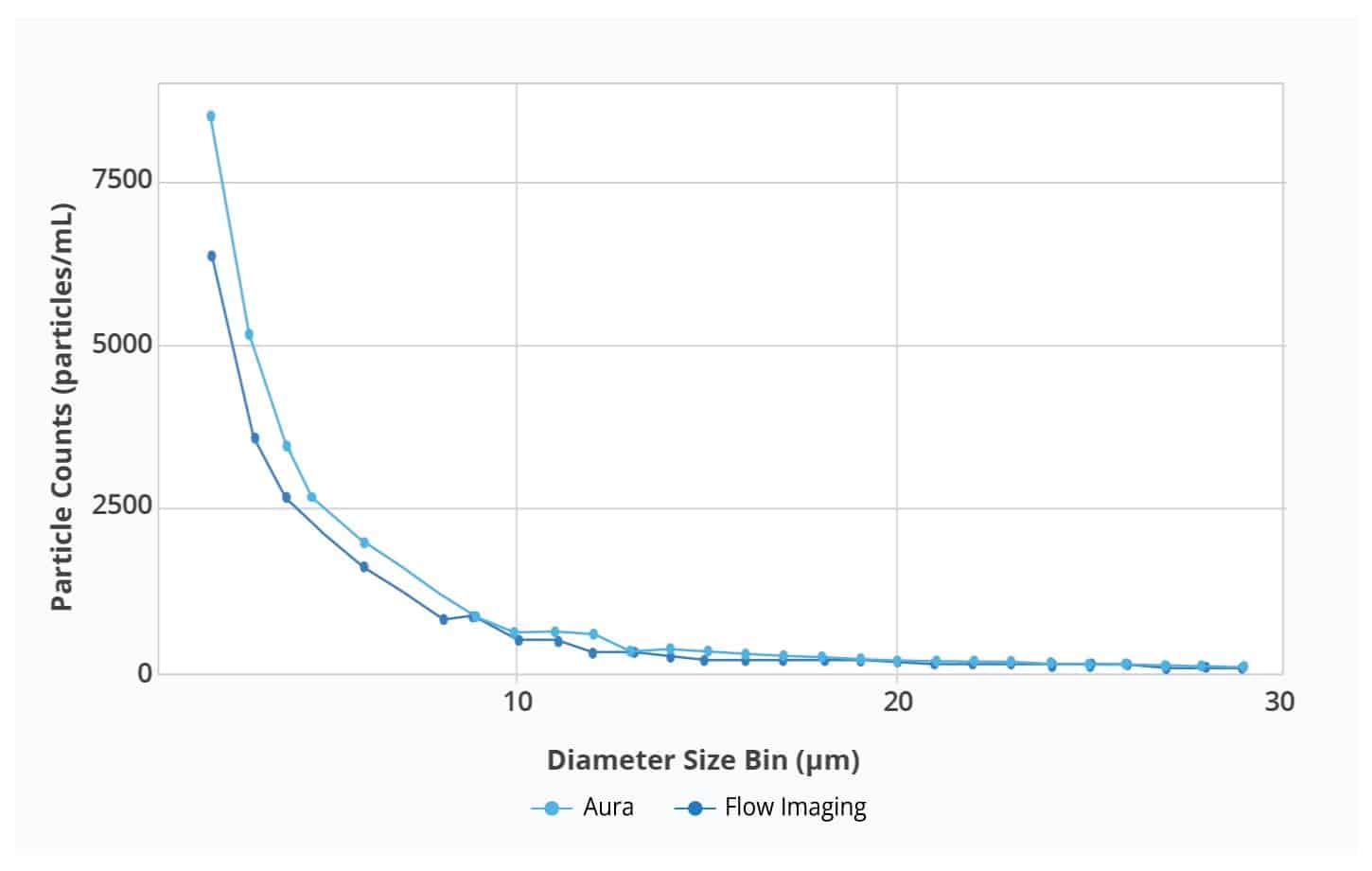
Particle size distributions as a function of counts for Aura and flow imager. Aura consistently detected more particles in every size bin compared to flow imager, with differences becoming more dramatic as particle size decreases.
Identify Your Best Formulations
Aura’s breakthrough technology makes it easier than ever to get to your best biologics formulation faster.
You no longer have to spend hours scrolling through images trying to differentiate between proteins and other non-protein aggregates—with Aura PTx, now you can ID them and find the “sweet spot” of your optimal formulation quickly, right out-of-the-box.
Analyze Aggregates with Low Volume and High Throughput
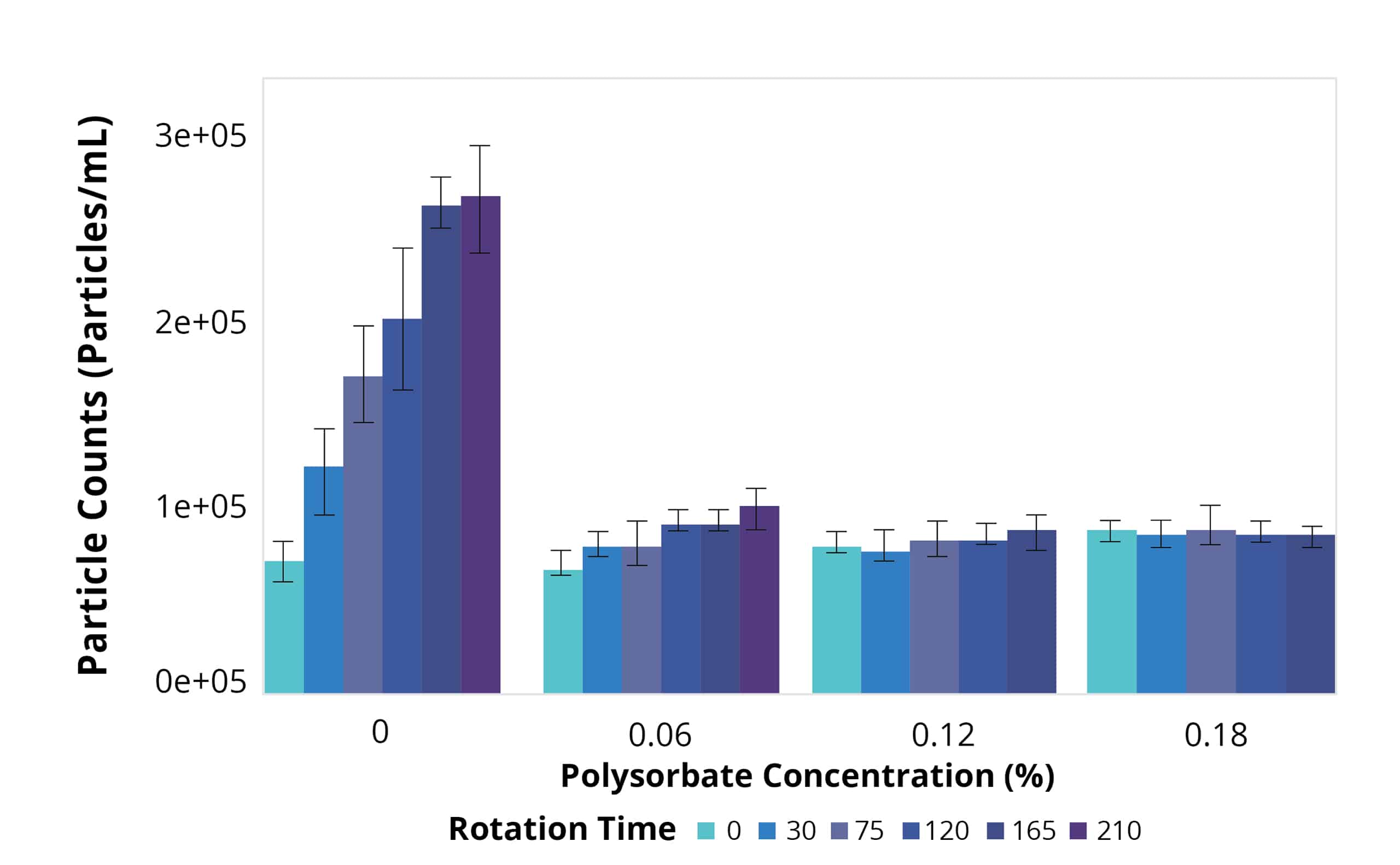
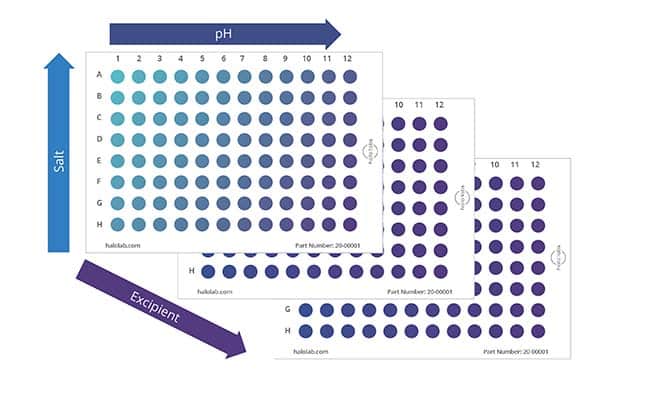
Today, many drugs are given subcutaneously at high concentrations and low volumes, heightening the importance of protein formulation development. At this stage, candidates are tested for their ability to dissolve in a wide range of salt and acidic environments, compatibility with the primary container, and stability in platform formulations subjected to representative stress conditions.
To obtain the most information with minimal samples used, Aura PTx provides a convenient solution through its automated 96-well format, which requires only 5 uL of sample per test. Analysis takes just 1 minute per sample so that multiple parameters can be rapidly assessed without any dilution or sample prep required. This streamlines the assessment of various formulation conditions efficiently.
Measure Highly Viscous Solutions
Analyzing highly viscous solutions during formulation development can be challenging, particularly when sample volume is low.
Traditional techniques such as Light Obscurity (LO) and Flow Imaging (FI) are not very sensitive to highly viscous products. This is why Aura provides a high refractive index contrast technique, which makes it possible to perform highly sensitive measurements of subvisible protein aggregates in the 2 μm – 4 mm size range, enabling 30% higher reproducibility.
Get More Sensitive Measurements
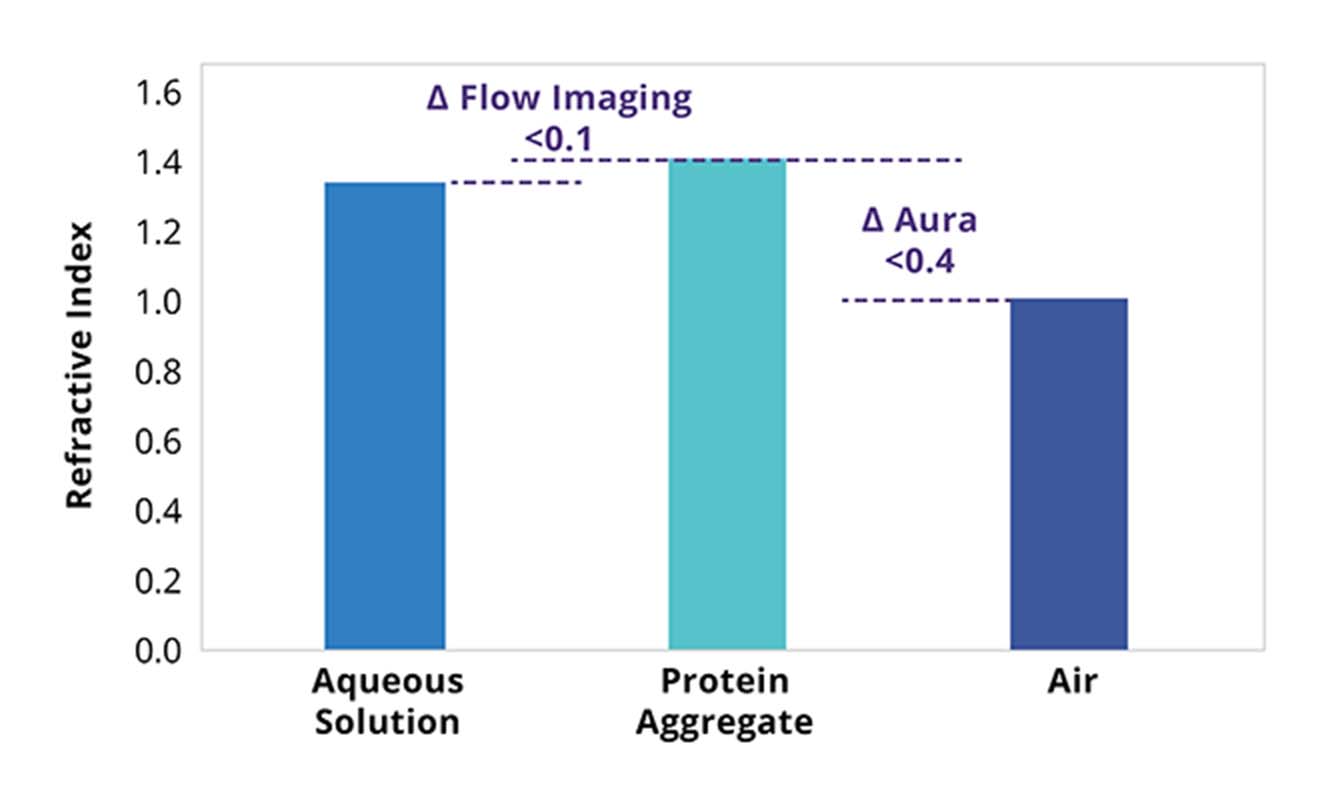
Flow imaging methods must contend with imaging microscopic translucent particles in liquid, which provide minimal refractive index contrast and can be susceptible to undercounting as well as undersizing protein aggregates.1
BMI by contrast, measures conducting image analysis on particles captured on a membrane surface. Imaging is done in air rather than liquid, which means the system is not subject to matrix effects or solution refractive index.
The end result is that even translucent particles can be imaged clearly, allowing for more precise measurements and characterization of particle size and shape during protein formulation development.
References
Zölls et .al Flow Imaging Microscopy for Protein Particle Analysis—A Comparative Evaluation of Four Different Analytical Instruments. AAPS J. 2013;15:1200–11.
Don't Settle for Flow Imaging
Protein stability and preventing aggregation are crucial for protein formulation development, but current subvisible analysis techniques may only be giving you part of the story. Incorporating Aura in comprehensive product stability studies can provide a more complete understanding of the factors affecting protein stability.
As biologic formulations reach higher levels of concentration, traditional particle analysis methods that require larger sample sizes may become unreliable when assessing subvisible aggregates due to the limited availability of material.
That's why it's time to leave flow cell imaging behind.
Featured products
Frequently Asked Questions
The development process of biologics involves several stages from discovery to commercialization, encompassing target identification, preclinical studies, clinical trials, and regulatory approval. Initially, target identification and validation involve identifying molecular targets (e.g., proteins, receptors) implicated in disease pathways and validating their therapeutic potential. Preclinical studies assess the safety, efficacy, and pharmacokinetics of candidate biologics in cellular and animal models to select lead candidates for clinical development. Clinical trials then evaluate the safety and efficacy of biologics in human subjects through progressively larger and more rigorous trials (Phase I, II, and III), culminating in regulatory submission for approval. Regulatory agencies review data on quality, safety, and efficacy to determine whether the biologic meets criteria for market authorization. Post-marketing surveillance monitors the ongoing safety and effectiveness of the biologic in real-world settings. Each of these steps is critical to a comprehensive development program ensuring the biologic's success.
Subvisible particles (SVP), which include particles larger than 1 micrometer in size, are tested using various analytical techniques to ensure the quality and safety of pharmaceutical products. Common methods for testing subvisible particles include microscopy techniques such as Backgrounded Membrane Microscopy (BMI) and Fluorescence Membrane Microscopy (FMM) to detect, count and characterize these particles, more effectively than traditional methods.